Technology
Conference

Introduction of COREX process
Essential Features
COREX consists of two reactors, the reduction shaft and the melter-gasifier. The reduction shaft is placed above the melter-gasifier and reduced iron bearing material descends by gravity. The volume of the reduction shaft and the melter-gasifier is about 600 m3 and 2200 m3 respectively.
Reduction Shaft:
Iron ore, pellets and additives (limestone and dolomite) are continuously charged into the reduction shaft via lock hopper system located on the top of the shaft. Some amount of coke is also added to the shaft to avoid clustering of the burden inside the shaft due to sticking of ore/pellets and to maintain adequate bed permeability. The reduction gas is injected through the bustle located about 5 meters above the bottom of the shaft at 850°C and over 3-bar pressure. The specific reduction gas flow is about 1200Nm3/ton of iron bearing burden charged to the shaft. The gas moves in the counter current direction to the top of the shaft and exits from the shaft at around 250°C. About 5-6% of coke is also added to the shaft to avoid clustering of the burden inside the shaft due to sticking of ore/pellets and to maintain adequate bed permeability. The iron bearing material gets reduced to over 95% metallization in the shaft and is termed as DRI. Subsequently, six screws discharge the DRI from the reduction shaft into the melter-gasifier. The metallization degree of the DRI and the calcination of the additives are strongly dependent on the following parameters:
§ Amount and quality of the reduction gas flow
§ Temperature of the reduction gas
§ Reducibility of the iron bearing burden
§ Average particle size and the distribution of the solids charged
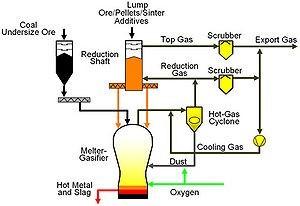
Schematic diagram of a COREX plant Melter-Gasifier: The melter-gasifier can largely be divided into three reaction zones § Gaseous free board zone (upper part or dome) § Char bed (middle part above oxygen tuyeres) § Hearth zone (lower part below oxygen tuyeres) Due to continuous gas flow through the char bed, there also exists a fluidized bed in the transition area between the char bed and the free board zone. The hot DRI at around 600-800 °C along with partially calcined limestone and dolomite are continuously fed into the melter-gasifier through DRI down pipes. The DRI down pipes are uniformly distributed along the circumference near the top of the melter-gasifier so as to ensure uniform distribution of material over the char bed. Additionally non-coking coal, quartzite and required quantity of coke are continuously charged by means of lock hopper system. The operating pressure, in the melter-gasifier is in excess of 3 bars. Oxygen plays a vital role in COREX process for generation of heat and reduction gases. It is injected through the tuyeres, which gasifies the coal char generates CO. The hot gases ascend upward through the char bed. The sensible heat of the gases is transferred to the char bed, which is utilized for melting iron and slag and other metallurgical reactions. The hot metal and slag are collected in the hearth. The efficiency of the furnace depends largely on the distribution of this gas in the char bed and utilization of the sensible heat of the gas. The dome temperature maintained between maintained between 1000°C to 1100°C, which assures cracking of all the volatile matter releases from the coal. The gas generated inside the melter-gasifier contains fine dust particles, which are separated in hot gas cyclones. The dust collected in the cyclones is recycled back to the melter-gasifier through the dust burners, where the dust is combusted with additional oxygen injected through the burners. There are four such dust burners located around the circumference of the melter-gasifier above the char bed. The gas from the melter-gasifier is cooled to the reduction gas temperature (850°C) through the addition of cooling gas. A major part of this gas is subsequently fed to the reduction shaft. The excess gas is used to control the plant pressure. This excess gas and the reduction shaft top gas are mixed prior to the take over point and is termed as COREX export gas. The export gas has high net calorific value of approximately 7,500 – 8000 kJ/m³ (STP). This gas is suitable for use for a wide range of applications like power generation etc. Corex is a two reactor but three-stage process. The blast furnace concept has been used, virtually splitting it into two at the cohesive zone interface. Accordingly a Corex plant has shaft unit, where iron ore pellets (with or without some closely sized lump ore) is reduced by gases emanating from the second unit to make hot sponge iron (first stage). This is mechanically transferred to the second unit or Melter-Gasifier where it is melted and carburised (second stage) by injection of both coal and oxygen. In the upper part of the Melter-Gasifier a fluidised bed of coal char is maintained (third stage), where any CO2 or H2O is converted to CO and H2. Since there is practically no CO2 or H2O in the gas leaving the Melter-Gasifier, we say that the degree of post combustion of Corex gas is zero, resulting in a gas rich in chemical energy. Reactions in Reduction Shaft: Following are the primary reactions taking place inside the reduction shaft: § Reduction of iron oxide by CO and H2 and transforming the iron oxides to metallic iron. Fe2O3 → Fe3O4 → FeO → Fe § Calcination of Limestone and Dolomite CaCO3 → CaO+CO2 (endothermic) CaCO3.MgCO3→CaO.MgO+2CO2 (endothermic) § Carbon deposition reaction and formation of Fe3C 2CO → CO2+C (exothermic) 3Fe+2CO → Fe3C+CO2 (exothermic) Out of the above mentioned reactions, reduction of iron oxide by H2 and calcination reactions are endothermic. On the other hand, reduction of iron oxide by CO gas and carbon deposition reactions are exothermic in nature. The reduction gas is almost fully desulphurized in the shaft due to the presence of the burnt lime and dolomite according to the following reactions: CaO + H2S → CaS + H2O MgO + H2S → MgS + H2O Low content of hydrogen sulphide of the top gas is important with respect to the further usage of the COREX gas. Reactions in Melter-Gasifier: Following are the reactions taking place in melter-gasifier: § Drying of Coal(100 oC) § Devolatilisation of coal (200 oC to 950 oC) and liberation of methane and higher hydrocarbons. § Decomposition of volatile matter Due to higher temperature prevailing in the melter-gasifier free board zone, the hydrocarbons are cracked intohydrogen and elementary carbon CnHm = nC + (m/2)H2 It is desirable that all higher hydrocarbons are cracked in the free board zone so as to assure generation of a good quality reduction gas. Maintaining dome temperature between 1000 oC to 1100 oC ensures the same. Other reactions in the free board zone are: § CO2 + C = 2CO (Boudouard Reaction) H2O + C = CO + H2 (Water Gas Reaction) CO + H2O = CO2 + H2 (Shift Reaction) § Decomposition of the undecomposed limestone and dolomite. § Residual Reduction of the iron oxide. § Direct reduction of FeO int the DRI takes place by carbon in the char bed. § Combustion of coal char by oxygen. Burning of the coal char takes place near the tuyeres. The maximum temperature inside the melter-gasifier exists in front of the tuyeres. The following carbon gasification reactions takes place in the tuyeres area. 2C + O2 = 2CO 2CO + O2 = 2CO2 C + CO2 = 2CO § Melting and formation of hot metal and slag respectively. The efficiency of the COREX process depends on the following parameters: § Size and chemical analysis of the raw especially the coal § Low C02 percentage in the reduction gas so as to ensure higher metallization of the DRI § Optimum distribution of oxygen between the tuyeres and dust burners § Permeability of the char bed § High system pressure § Higher melting rate operation
Process Chemistry
World Scenairon
§ Development of the COREX process dates back to the 1980s and was performed by Voest-Alpine (Germany/Austria). The first commercial COREX unit was constructed between 1985 and 1987 at ISCOR’s Pretoria works, after first testing the process in Germany. The plant had various problems (due to inexperience) after the start-up in December 1987. After reconstruction and de-bugging, the plant has been successfully in operation since 1989 and was given over to ISCOR(capacity 300,000-ktpa). Reconstruction of some parts and new operation conditions improved the performance greatly, leading to production of high quality iron, and high productivity and availability. The plant demonstrated to be economically attractive, with 30% lower production costs than the blast furnace on site, despite the low capacity of the COREX unit. The clean excess fuel gas is used on site in furnaces and coke ovens. The COREX process proved to be very flexible(with respect to the fuel rate, and additives addition), insensitive to high alkali content of the ore (and burden), and easy to operate.
§ The preliminary success of the first COREX plant lead to the decision to build a larger COREX (C-2000, 650,000 tonnes per year) in South Africa by Saldanha Steel, a subsidiary of ISCOR. C-2000 means nominal production rate is 2000 tons of hot metal per day. Saldanha Steel is now a part of Mittal Steel South Africa which in turn is part of global steel company Arcelor-Mittal. In 1998, ISCOR decided to close the Corex-based Pretoria plant due to an “unprofitable economic situation”. The off-gases of the new COREX unit will be used to produce 800,000 tonnes per year of DRI, following a similar design to that at HANBO Steel, South Korea. This decision also seems to be based on the environmental performance of the COREX process, as the site is located near a nature preserve. This plant, which includes a thin slab caster, began operation in January 1999.
§ Since 1995 a COREX plant has been in operation in the Republic of Korea, with twice the capacity of the South African plant (C-2000). South Korea’s Pohang Iron and Steel Co. has a 600,000-700,000 ton per year unit COREX plant. Several more orders have been placed for C-2000 plants in India, South Korea, and South Africa.
§ The Chinese steel producer Baosteel Pudong Iron and Steel Co. Ltd. (For Short Pudong steel) at Luojing, near Shanghai started up a Corex C-3000 plant in early November 2007, with a nominal production capacity of 1.5 million tons of hot metal per year(or 3000 thm/day). The project was completed under the management of Siemens Metals Technologies within a period of 29 months.
§ The latest generation of Corex plants, the C-3000, is ideally suited for integration into green- or brown-field steel works projects. It can replace the blast furnace, or can be used as a source of virgin iron for minimills. The economics of the Corex plant already provide an answer to future scrap and coke shortages, and the continually increasing demands placed on steel quality. Another alternative is the installation of a Corex C-3000 plant as a stand-alone merchant plant for the production of hot metal and/or pig iron.

World's Largest Corex Ironmaking Plant setup by the Chinese steel producer Baosteel Pudong Iron and Steel Co. Ltd.
Tianjin Over World Non Coke Iron Making Technical Consultancy Co.,Ltd. All Rights Reserved
Tel.:+86-22-24410619 Fax:+86-22-24410619
TJ ICP 1100023 Email:info@driinfo.com